U.S. Announces Major Overhaul of Childhood Vaccine Schedule, Cutting Universal Shot Recommendations
Federal health authorities reduce routine immunization guidance for six diseases while preserving core vaccinations and insurance coverage
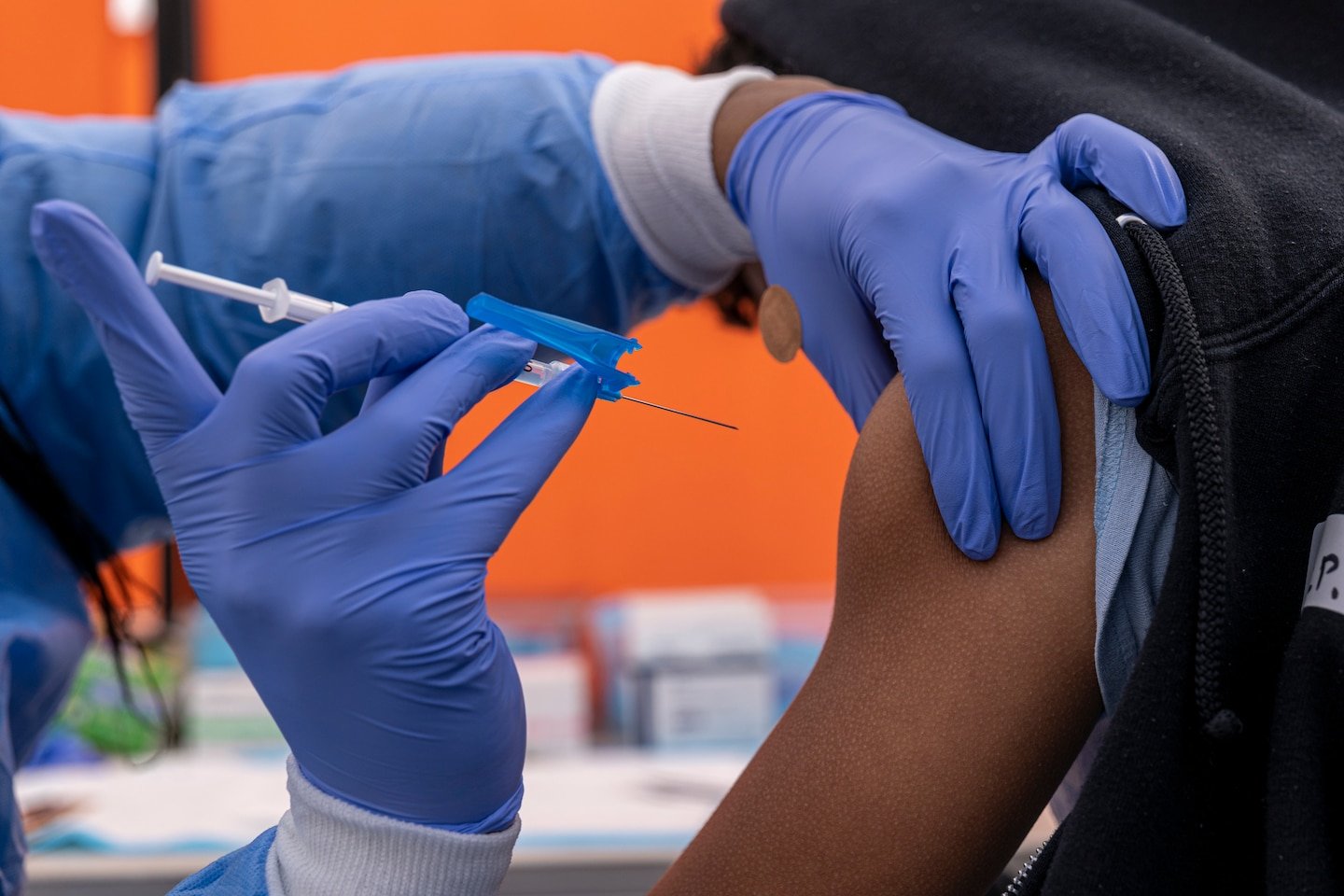
The United States has unveiled a sweeping revision to its federal childhood immunization schedule that will reduce the number of vaccines universally recommended for all children, marking one of the most significant shifts in national vaccination policy in decades.
Under new guidance issued on January 5, the Centers for Disease Control and Prevention and the Department of Health and Human Services dropped universal recommendations for immunization against several diseases, including influenza, rotavirus, some forms of meningococcal disease, respiratory syncytial virus (RSV), hepatitis A and hepatitis B, for most children, instead advising that these vaccines be administered only to high-risk groups or in consultation between parents and healthcare providers.
This “shared clinical decision-making” approach is intended to more closely align U.S. practice with policies in other wealthy nations, where fewer vaccines are routinely recommended for all children.
Core immunizations against diseases such as measles, polio, whooping cough, tetanus and human papillomavirus will continue to be recommended for all children, and insurers have stated that coverage for all previously recommended vaccines will remain intact under private plans, Medicaid, the Children’s Health Insurance Program and other public programmes.
Officials framed the changes as an effort to rebuild public trust in vaccination and increase transparency following the COVID-19 pandemic, when confidence in public health institutions declined.
Health authorities noted that many peer nations recommend fewer routine immunizations, and the new schedule reflects an assessment of disease risks and international practices.
The update also alters the schedule for the human papillomavirus vaccine, moving from a two-dose to a single-dose recommendation for most children.
Public health organisations and medical experts have sharply criticised the revision, warning that it could sow confusion among parents and providers, reduce overall vaccination rates, and contribute to the resurgence of vaccine-preventable diseases.
They emphasise that differences in healthcare systems, disease prevalence and access to care between the United States and other countries make direct comparisons challenging.
The overhaul was promulgated without the customary review by the Advisory Committee on Immunization Practices, prompting concerns about the transparency and scientific basis of the process.
Under new guidance issued on January 5, the Centers for Disease Control and Prevention and the Department of Health and Human Services dropped universal recommendations for immunization against several diseases, including influenza, rotavirus, some forms of meningococcal disease, respiratory syncytial virus (RSV), hepatitis A and hepatitis B, for most children, instead advising that these vaccines be administered only to high-risk groups or in consultation between parents and healthcare providers.
This “shared clinical decision-making” approach is intended to more closely align U.S. practice with policies in other wealthy nations, where fewer vaccines are routinely recommended for all children.
Core immunizations against diseases such as measles, polio, whooping cough, tetanus and human papillomavirus will continue to be recommended for all children, and insurers have stated that coverage for all previously recommended vaccines will remain intact under private plans, Medicaid, the Children’s Health Insurance Program and other public programmes.
Officials framed the changes as an effort to rebuild public trust in vaccination and increase transparency following the COVID-19 pandemic, when confidence in public health institutions declined.
Health authorities noted that many peer nations recommend fewer routine immunizations, and the new schedule reflects an assessment of disease risks and international practices.
The update also alters the schedule for the human papillomavirus vaccine, moving from a two-dose to a single-dose recommendation for most children.
Public health organisations and medical experts have sharply criticised the revision, warning that it could sow confusion among parents and providers, reduce overall vaccination rates, and contribute to the resurgence of vaccine-preventable diseases.
They emphasise that differences in healthcare systems, disease prevalence and access to care between the United States and other countries make direct comparisons challenging.
The overhaul was promulgated without the customary review by the Advisory Committee on Immunization Practices, prompting concerns about the transparency and scientific basis of the process.